Cytoskeleton
The cytoskeleton is a mesh of different proteins in cells. It was for a long time believed to be a characteristic feature of cells with a nucleus (eukaryotic cells) like all cells from multicellular organisms like fungi, plants and animals. Recently it became clear that even cells without a nucleus (prokaryotic cells) like bacteria have proteins that form a cytoskeleton. Here we concentrate on eukaryotic cells.In the course of the human genome project more than 800 probably cytoskeleton related genes were found. There are three major components of the cytoskeleton: microtubules, intermediate filaments and microfilaments.
The typical construction in a non-dividing cell in culture is as follows: a thin dense actin-cortex is supporting the plasma-membrane like an underlying shell. Actin stress fibers span through the cell starting from the focal adhesion points, where the cell is attached to the ground. Microtubules radiate from microtubule organizing centers throughout the cytoplasm and are associated with intermediate filaments.
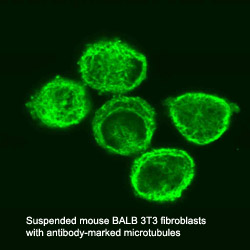
Microtubules
consist of two different globular protein subunits, alpha-tubulin and
beta-tubulin. One alpha- and one beta-tubulin form a heterodimer. Long
chains of these heterodimers compose protofilaments, wherein always a
alpha-tubulin is followed by a beta-tubulin. In singlet microtubules
generally 13 protofilaments associate site by site to form a tube of 25
nm diameter. In some special structures doublet (in cilia and flagella)
and even triplet (in centrioles and basal bodies) microtubules exist,
having a 13-protofilament microtubule accompanied by one or two
10-protofilament ones.
Microtubules assemble and disassemble dependent on the temperature and
the surrounding tubulin concentration. Every microtubule has a (-) and
a (+) end. At the (+) or beta-tubulin end new heterodimers are added
faster and at lower tubulin concentrations than at the (-) or
alpha-tubulin end. When the critical tubulin concentration for the (+)
end polymerisation is reached but not the higher critical concentration
for the (-) end, the (-) end depolymerises while heterodimers at the
(+) end are added, i.e. the microtubule treadmills. The alpha-tubulin
as well as the beta-tubulin subunit binds a small guanosine
triphosphate (GTP). The GTP bound to the alpha-tubulin faces the
beta-tubulin subunit of the heterodimer, while the GTP of the
beta-tubulin subunit directs away from the heterodimer. When a new
dimer is incorporated into a microtubule the beta-tubulin bound GTP is
hydrolysed to guanosine diphosphate (GDP). If the polymerisation is
faster than the hyrdolysation, a GTP-cap occupies the (+) end and
causes the microtubule to depolymerise faster than polymerise.
In a cell the dynamics of microtubules are regulated by
microtubule-associated proteins (MAPs). Some MAPs stabilize
microtubules, while others destabilize microtubules.
Stabilizing MAPs have a microtubule-binding domain and an acidic
projection domain, which can bind to intermediate filaments or
membranes and is suspected to determine the distance in between bundled
microtubules. Such bundles are for example formed in axons with the
help of the MAP tau. The ability of MAPs to bind microtubules can be
altered by phosphorylation of the MAPs by MAP kinases.
Destabilizing MAPs are for example katanin, which breaks bonds between
tubulin subunits, or Op18, which increases the disassembly frequency of
microtubules presumably by reducing the pool of available heterodimers.
The arrangement of microtubules in cells is determined by
microtubule-organizing centers (MTOCs). These MTOCs consist of
different proteins like gamma-tubulin and pericentrin. The microtubules
direct their (-) end to the MTOC. In general every eukaryotic cell has
a primary MTOC the centrosome.
An important role of microtubules is providing a pathway for
intracellular movements of organelles and proteins. This is done by
motor proteins (kinesins and dyneins) under consumption of adenosine
triphosphate (ATP). Most kinesins carry their cargo along microtubules
in (+) direction, while dyneins do so in (-) direction. These proteins
have two head-domains, which are alternating attached to the
microtubule and bend before the other head attaches, so that a steady
movement along a protofilament is generated. The tail domain of the
kinesin appoints the kind of cargo that can be bound and transported.
Dynein can bind its cargo not directly to its tail domain but needs the
protein dynactin for mediation.
Some intermediate filaments
(IF) are homopolymeres of one protein, some are heteropolymeres of two
or more proteins. All intermediate filament proteins (67 human genes
known) have a common basic structure with main differences at both
ends. They form homogenous, apolar fibers with diameters of 10-12 nm.
Some IF proteins are ubiquitous (e.g. vimentin) others are restricted
e.g. to neurons of the central nervous system (neurofilament proteins),
muscle (desmin, syncoilin) or epithelial cells (keratin). As well as
the expression the organisation of the intermediate filaments is
cell-type-dependent. In many epithelial cells e.g. filaments are
distributed all over the cytoplasm and attach to the nucleus, while in
primary fibroblasts vimentin is orientated towards the periphery and
spans neither the whole cytoplasm nor is it connected to
cell-cell-adhesion sites.
The intermediate filaments network is dynamically influenced by a bunch
of other proteins. The IFs provide mechanical stability as well as they
take part in the assembly of the nuclear envelope or neurofilaments
contribute to the radial growth of neurons.
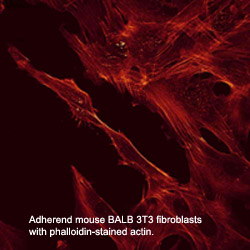
Microfilaments
are variable in length and their diameter is about 7-9 nm. These
filaments are cross-linked into networks or bundles. In most cases a
shell of microfilaments supports the plasma membrane.
Microfilaments are a polymer of actin protein subunits plus attached
proteins like cross-linkers. Most multicellular organisms have several
actin isoforms. Humans have six actin genes, four encode alpha-actin,
one beta- and one gamma-actin. alpha-actin is found in muscle cells
where it plays an important role in contracting the cell. Whereas
beta-actin is localized in the front of moving cells and gamma-actin
forms stress fibers. Actin protein as a polymer without attached
proteins is called filamentous actin (F-actin), while the globular
actin monomers are called G-actin. The actin subunits are structured in
two lobes with a cleft in between, where a magnesium ion (Mg+) and an
ATP are located. After a G-actin is incorporated into a filament, the
ATP is hydrolysed to ADP. The assembly of F-actin is described at Actin Dynamics and Kinetics.
There are a lot of proteins that regulate the actin assembly, filament
length and stability in vivo. Other proteins promote branching or
linking of actin filaments into networks. And last but not least a
family of proteins called myosins, which consist of an ATPase active
head domain and a specific tail region, can move along microfilaments.
These motor proteins can transport membrane vesicles or cause
contractions not only in muscle cells but also in other cells.
The functions of the cytoskeleton are manifold and do not only sustain the cell's shape and its mechanical scaffold. The semiflexible microfilaments make cells mobile, help them to divide in mitosis (cytokinesis) and are responsible for muscular contraction. The relatively stiff microtubules play an important role highway for transport of vesicles and organelles and in the separation of chromosomes during mitosis (karyokinesis). The flexible intermediate filaments strengthen the cell additionally. It seems that the cytoskeleton is also involved in signalling across the cell.
Taking these functions into account it is plausible that the disruption of the cytoskeleton or even subtle changes of its integrity may cause pathological outcomes.
- References
- Coulombe,P.A., L.Ma, S.Yamada, and M.Wawersik. 2001. Intermediate filaments at a glance. J. Cell Sci. 114:4345-4347.
- Iida,J.,
T.J.Itoh, H.Hotani, K.Nishiyama, H.Murofushi, J.C.Bulinski, and
S.Hisanaga. 2002. The projection domain of MAP4 suppresses the
microtubule-bundling activity of the microtubule-binding domain. J.
Mol. Biol. 320:97-106.
- Lodish,H., A.Berk,
P.Matsudaira, C.A.Kaiser, M.Krieger, M.P.Scott, S.L.Zipursky, and
J.Darnell. 2003. Molecular cell biology. W.H.Freeman, New York.
- Mayer,F. 2003. Cytoskeletons in prokaryotes. Cell Biol. Int. 27:429-438.
- Srivatava,R.K.,
M.P.Mattson, and D.L.Longo. 2001. Cytoskeletal Involvement in
Apoptosis. In Programmed cell death, Volume 1. M.P.Mattson, S.Estus,
and V.Rangnekar, editors. Elsevier, Amsterdam; London; New York;
Oxford; Paris; Shannon; Tokio. 237-267.
- Tokuraku,K., M.Katsuki, T.Matui, T.Kuroya, and S.Kotani. 1999. Microtubule-binding property of microtubule-associated protein 2 differs from that of microtubule-associated protein 4 and tau. Eur. J. Biochem. 264:996-1001.